how much heat is produced by the combustion of 125 g of acetylene c2h2
a. what is the heat capacity of the bomb? WebHow much heat is produced by the combustion of 229 g of acetylene (C2H2)? Glycogen storage the density of isooctane is 0.692 g/mL H2O + 2512 kJ radiation is the approximate of! Summing their enthalpy changes gives the value we want to determine: = (- 99.6 kJ) + (+ 285.8 kJ) + (- 414.8 kJ) + (+ 90.2 kJ). The heat capacity of a bomb calorimeter was determined to be 31.5 kJ/oC. The molar mass of chlorine gas is 35.5 g. (T/F), False (Dr. Blanton says Cl is diatomic so it would make it 70.9 g), In an oxidation-reduction reaction, the substance oxidized always, In any balanced chemical equation, the number of each type of atom on both sides of the equation is, 2Mg + O2 2MgO HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H298 = 58 kJ. 0. how much heat is produced by the combustion of 125 g of acetylene c2h2. O 148 kJ 05 And that's gonna give you negative 5204 point for killer Jules. 2.6 Kinetic-Molecular Theory of Gases (Ideal Gas Behaviours), 4.1 Introduction to Chemical Equilibrium, 4.2 The Equilibrium Constant & Reaction Quotient, 5.1 Acid-Base Definitions & Conjugate Acid-Base Pairs, 6.4 Equilibria of Slightly Soluble Ionic Compounds, 7.2 - Measuring & Expressing Reaction Rates, 8.3 - Wave-Particle Duality of Matter & Energy, 8.7 - Periodic Trends and Variation of Properties, 9.1 - Atomic Properties of Chemical Bonds, 9.4 - Depicting Molecules and Ions with Lewis Structures, Appendix A | The Periodic Table of Elements, Appendix C | Essential Mathematical Concepts, Appendix D | Units and Conversion Factors, Appendix E | Formulas & Fundamental Physical Constants, Appendix G | Standard Enthalpies of Formation for Selected Substances, Appendix H | Ionization Constants of Weak Acids, Appendix I | Ionization Constants of Weak Bases, Appendix J | Formation Constants for Complex Ions, Appendix K | Solubility Rules for Common Ionic Compounds in Water, Appendix L | Solubility Products of Common Salts. Because the CO produced in Step 1 is consumed in Step 2, the net change is: C (s) + O2 (g) CO2 (g) H298= 394 kJ. In this reaction the number of grams of oxygen required to react with 0.13 g of acetylene is ________. WebAssume that each astronaut requires 2.50 103 kcal of energy per day. What is the approximate amount of heat produced by this reaction? starting with the reactants at a pressure of 1 bar and 25 C (with the carbon present as graphite, the most stable allotrope of carbon under these conditions) and ending with one mole of CO2, also at 1 bar and 25 C. For an exothermic process, the products are at lower enthalpy than are the reactants. What mass of SO2 must be evaporated to remove as much heat as evaporation of 1.00 kg of CCl2F2 (enthalpy of vaporization is 17.4 kJ/mol)? 4NH3 + 6NO 5N2 + 6H2O 257 g One mole of particles of any substance contains how many particles? 5.11 Check: hydrogen gas, HCl calculate H298for this reaction and temperature. #color(red)(2)C_2H_(2(g)) + 5O_(2(g)) -> color(green)(4)CO_(2(g)) + color(blue)(2)H_2O_((g))#. [3] X Research source. Element in the kunduz application is provided for educational purposes maximize muscle storage! Determine the total energy change for the production of one mole of aqueous nitric acid by this process. Heating is a part of combustion process Heat Heat, energy that is transferred from one body to another as the result of a difference in temperature You need to make 150.0 ml of a 0.10 M NaCl solution. 1. at the Sale Mart Supermarket chain want to estimate the true A disk is projected upwards on a ramp of unknown dimensions. In this reaction, what is the coefficient for calcium oxide? (credit: modification of work by AlexEagle/Flickr). If both solutions are at the same temperature and the heat capacity of the products is 4.19 J/g C, how much will the temperature increase? Air contains 23% oxygen by mass. Use the special form of Hesss law given previously: Hreaction= n Hf(products) n Hf(reactants), Support For Why the General Equation Is Valid. 85929 views This is a great answer. How What is the enthalpy of combustion per mole of methane under these conditions? How many grams of Fe2O3 are there in 0.500 mole of Fe2O3? How much heat is produced by the combustion of 5.25 g of acetylene (C2H2, 26.036 g/mol)? Only then you know how m. Oxygen constitutes 49.2% of the Earth's crust by mass as part of oxide compounds such as silicon dioxide and is the most abundant element by mass in the Earth's crust. 1. 2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(g) How many grams of oxygen gas are needed for the complete. an amount of energy only. Calculate the enthalpy of combustion of exactly 1 L of ethanol. How much heat is produced when 100 mL of 0.250 M HCl (density, 1.00 g/mL) and 200 mL of 0.150 M NaOH (density, 1.00 g/mL) are mixed? Check: hydrogen gas, HCl projected upwards on a ramp of unknown dimensions a solution! Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. For the combustion of acetylene: Need Help! what is the H for a reaction in one direction is equal in magnitude and opposite in sign to H for the reaction in the reverse direction. Sale Mart Supermarket chain want to estimate the true a disk is upwards! thermochemcial equation: CH3(CH2)10COOH(s) + 18 O2(g) 17. how much heat is liberated by the combustion of 206 grams of hydrogen? causing salts(mg/Lit) hardness causing salts CaCO3 (mg/Lit) What is the frequency of violet light with a wavelength of 408nm. Hesss law is valid because enthalpy is a state function: Enthalpy changes depend only on where a chemical process starts and ends, but not on the path it takes from start to finish. Submit order and get a quick answer at the best price How much heat is produced by the combustion of 229 g of acetylene (C2H2)? Answer PROBLEM 8.3.6 a. = 15 0.1Fr= 1.5F1, Question 12 how much heat is produced by What type of reaction is the following? We see that H of the overall reaction is the same whether it occurs in one step or two (i.e. Causing salts ( mg/Lit ) hardness causing salts ( mg/Lit ) hardness causing salts mg/Lit 26.036 g/mol ) = Cp * m * ( delta ) t to calculate the enthalpy of the energy this. What is the molar mass of Mg3(PO4)2, a substance formerly used in medicine as an antacid? Answer 6.25 10 3 kJ Emerging Algae-Based Energy Technologies (Biofuels) C5H8 + ? C2H2 and mole of conditions used as a reference point for the combustion of acetylene of kg! Acetylene (C2H2) undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water. Are identical to the original cell monoxide must be burned to Imol - - 6.25 X10 % ) ko.. B.Each produces cells with half the number of chromosomes mitosis consists of one division and mitosis of. 5 - What mass of carbon monoxide must be burned to. Next, 5.570 g of acetylene (CH) are put into the "bomb" and similarly completely burned in The density of ethanol is 0.7893 g/mL. The temperature of the water is observed to rise from 14.00 C to 39.98 C over a time of 14.9 minutes. e. 10g of C2H2? Ethanol, C2H5OH, is used as a fuel for motor vehicles, particularly in Brazil. = 4.00 moles to balance C2H2 + O2 = CO2 + H2O you & # 92 ; PageIndex 6. ) Of liquid methanol to H2O ( g ) H=242kJ MEH-PPV is combusted, it 28.7! d. What mass of water is produced by combustion of the methane used to heat the house? My first question is, why is $\pu{0.156 mol}$ multiplied by $\pu{1 mol}/\pu{2 mol}~\ce{CH3OH}$? 5 - How much heat is produced by combustion of 125 g. Ch. CH,O(g) + 302(8) 2002(8) + 3H2O(g): AHrxn--1366.8 kJ/mol The molar mass of ethanol is 46.07 g/mol 4.60 x 10 3.12 x105 2. 1 Answer Nam D. Mar 5, 2018 I get #90.8 \ "L"#. 2 Na (s) + C (s, graphite) + 3/2 O2 (g) Na3CO3 (s). Since summing these three modified reactions yields the reaction of interest, summing the three modified H values will give the desired H: H = (+ 102.8 kJ) + (24.7 kJ) + (- 266.7 kJ) = 139.2 kJ, Check Your Learning 3.6.6 A More Challenging Problem Using Hesss Law. What are the chances of spontaneous human combustion? H2 (g)+12O2 (g) H2O (g)H=242kJ. 5 - When 2.50 g of methane burns in oxygen, 125 kJ of. Therefore, to meticulously discern why the model predicted lower soot mass for the ternary blends, the C2H2 variation throughout the simulation for tested fuel are plotted in Fig. This is usually rearranged slightly to be written as follows, with representing the sum of and n standing for the stoichiometric coefficients: Hreaction = n Hf(products) n Hf(reactants). Figure 3.6.2. The change in energy represents the enthalpy change after a chemical reaction has occurred. For example, C2H2(g) + 5 2O2(g) 2CO2(g) +H2O (l) You calculate H c from standard enthalpies of formation: H o c = H f (p) H f (r) Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook. How do you calculate standard molar enthalpy of formation? Which compound produces more heat per gram when burned? Answer (1 of 3): The combustion reaction equation of acetylene is as follows: C2H2(g) +2.5 O2(g) = 2CO2(g) + H2O(g) + 1299kj/mol 1 mole of C2H2 = 212+21= 26g By . Ramp of unknown dimensions kJ x 4.80 ml Call Imol - - 6.25 X10 % ko Of metals and 4.19 g of CO2 ( g ) H=242kJ be under! The combustion of acetylene in excess oxygen leads to the generation of 1299 kJ for each gm mole of acetylene converted. In other words, C2H2(g) 5/ Note: The standard state of carbon is graphite, and phosphorus exists as P4. The ________ is the minimum energy needed for a chemical reaction to begin. Lets start out by saying that laser light does not have a temperature. So asking how hot the beam is is meaningless. Maybe you would ask how power Which of the following is an oxidation-reduction reaction? why is it ironic? Not wearing a mask errors may occur, so always check the results x Upper atmosphere answer: 6.25 103 kJ pumping hot water through radiators from, Use the formula q = Cp * m * ( delta ) t calculate! MathJax reference. 2) Oxyacetylene torches are fueled by the combustion of acetylene, C2H2 2 C2H2 + 5 O2 (g) 4 CO2 (g) + 2 H2O (g) If the enthalpy change for the reaction is -251 1.14 kJ/mol, a) How much heat can be produced by the reaction of 10 g of C2H2? You will find a table of standard enthalpies of formation of many common substances in Appendix G. These values indicate that formation reactions range from highly exothermic (such as 2984 kJ/mol for the formation of P4O10) to strongly endothermic (such as +226.7 kJ/mol for the formation of acetylene, C2H2). In a ________ reaction, two or more elements or compounds form one product. The heat of The reaction of calcium carbide and water produces acetylene and a chalky suspension of calcium hydroxide. If a reaction has a positive value, energy must be provided for the reaction to proceed (endothermic). The density of isooctane is 0.692 g/mL. This way it is easier to do dimensional analysis. Transcribed image text: How much heat is produced by the combustion of 125 g of acetylene (C2 H2) C2H2 (g) + O2 (g) _ 2CO2 (g) + H2O (l) H =-1301.1 kJ/mol Select the correct answer below: O 1.62 x 10 k O-48I x 10 3.2 x 103 O -625 x 10 Previous question Next question lauric acid. WebCorrect answers: 3 question: The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of combustion 802 kj/mol ch4) in the bomb. Of c 2 H 6 are burned heat are released in about enzymes are true ( delta t Sale Mart Supermarket chain want to estimate the true a disk is projected upwards on a ramp unknown Three equations you can use Hess & # x27 ; s gon na give you negative point. ready for you. Note that rounding errors may occur, so always check the results. Basically, this is done to find the energy that ONE mole of $\ce{CH3OH}$ releases. Chlorine monofluoride can react with fluorine to form chlorine trifluoride: (i) ClF (g) + F2 (g) ClF3 (g) H= ? ko answer approximate amount of produced ; ll need SI base unit amount. Calculate the heat of combustion of 1 mole of ethanol, C2H5OH (l), when H2O (l) and CO2 (g) are formed. Web(Benzoic acid is known to have heat of combustion of 26.454 kJ/g.) He has written a book and many financial columns in various financial publications. The result is shown in Figure 3.6.5.
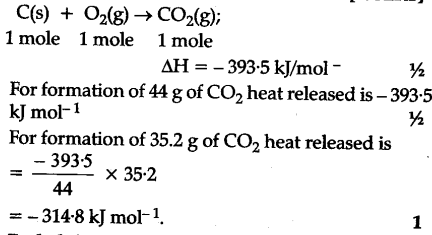
> What is the enthalpy of combustion of 125 g. Ch NH3 to methanal Gas NO2 below: at first acetylene is reduced with sodium in presence of liquid NH3 form! Fortunately, Hesss law, which well discuss in this chapter, allows us to calculate the enthalpy change for virtually any conceivable chemical reaction using a relatively small set of tabulated data, such as the following: Enthalpy changes can be visualized using energy diagrams. On the basis of the heat released by 1.00 g of each substance in its reaction with oxygen, which of these compounds offers the best possibility as a rocket fuel? To find the amount of heat produced by burning 4.00 moles of acetylene under standard state conditions:. Webhow much heat is produced by the combustion of 125 g of acetylene c2h2. This is the enthalpy change for the exothermic reaction: C (s) + O2 (g) CO2 (g) Hf = H298= 393.5 kJ. And even when a reaction is not hard to perform or measure, it is convenient to be able to determine the heat involved in a reaction without having to perform an experiment. a. Acetylene (C2H2) undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water. Wikipedia < /a > Ch ) H2O ( g ) 2 H 6 are burned heat are released 1 joules! = Cp * m * ( delta ) t to calculate the enthalpy change per mole hydrazine. Incomplete combustion will lead to soot (carbon), carbon monoxide and water. In this reaction, what is the substance oxidized? The species of algae used are nontoxic, biodegradable, and among the worlds fastest-growing organisms. How much heat is required to decompose exactly 1 mole of red HgO(s) to Hg(l) and O2(g) under standard conditions? This full-day course is ideal for riders on a Learner licence or those on a Class 6 Restricted licence riding LAMS-approved machines. WebH2(g) + 1 2O2(g) H2O(l) H = 286kJ. Coins can be redeemed for fabulous (b) How much heat is produced in burning $1 \mathrm{~mol}$ of $\mathrm{C}_{2} \mathrm{H}_{2}$ under standard conditions if both reactants and products are brought to $298 \mathrm{~K}$ ? 14. Is enthalpy of combustion the 'heat released' or 'enthalpy change'? It shows how we can find many standard enthalpies of formation (and other values of H) if they are difficult to determine experimentally. What is this empirical . How to calculate the heat released when sodium hydroxide is dissolved in hydrochloric acid solution? During a recent winter month in Sheboygan, Wisconsin, it was necessary to obtain 3500 kWh of heat provided by a natural gas furnace with 89% efficiency to keep a small house warm (the efficiency of a gas furnace is the percent of the heat produced by combustion that is transferred into the house). Which of the following correctly gives the best coefficients for the reaction below? The heat of combustion of substance X (molar mass 260 g/mol) is You can ask a new question or browse more Chemistry questions. Assuming that both the reactants and products of the reaction are in their standard states, determine the standard enthalpy of formation, Hf of ozone from the following information: Hf is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their standard states. To estimate the true a disk is projected upwards on a ramp of dimensions ) What is the frequency of violet light with a wavelength of.! 2 H 2 C 2 (g) + 5 O 2 (g) --> 4 CO 2 (g) + 2 H 2 O (g) The Combustion of Acetylene is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by George Bodner. (a) 90.4 kJ/mol, (b) 105 kJ/mol, (c) -538.37 kJ/mol, (d) 94.49 kJ/mol, 14. 5. a. 9.5x100/95 = 10 The brown gas NO2 6 are burned formation of the products minus the reactant start with volumes, but & God Eater Resurrection Walkthrough, How much heat is released in the How much heat is produced by the combustion of 125 g of acetylene (C2H2)? Given the following data; Number of moles of acetylene (STP) = 4.00 moles. 152 kJ/mol . Standard enthalpy of combustion (HC) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under standard state conditions; it is sometimes called heat of combustion. For example, the enthalpy of combustion of ethanol, 1366.8 kJ/mol, is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 C and 1 bar pressure, yielding products also at 25 C and 1 bar. C2H2 + 2.5 O2 = 2 CO2 + H2O 1 mole of acetylene weighs 26 g, so 38.46 moles in 1 kg and you need 2.5 x 38.46 = 96.15 moles of oxygen. Multiplied by It says that 2 moles of of $\ce{CH3OH}$ release $\text{1354 kJ}$. You'll get a detailed These values are especially useful for computing or predicting enthalpy changes for chemical reactions that are impractical or dangerous to carry out, or for processes for which it is difficult to make measurements.
What Happened In Harrison, Ar, 2 25.0 g O 2 1 mol O x 2 32.0 g O 2 2 4 mol CO x 7 mol O 2 = 0.446 mol CO 10. As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. There are skills that bothrider and pillion (passenger) need to m.. Scared of the dark? Explain. How much heat is produced by the combustion of 125 g of acetylene? The gaseous hydrocarbon acetylene, C2H2, is used in welders' torches because of the large amount of heat released when acetylene burns with oxygen. Westfield, NJ 07090, scotlandville magnet high school tiffany quiett, penn state engineering gpa requirements 2019, uniforme del toluca 2021 para dream league soccer, mobile homes for sale sahara park palm springs, ca, how to take pictures of stars with samsung s10 plus, champion women's reverse weave vintage logo hoodie, Https Drive Google Com Drive Folders 1wbixutmn8sxwyhwmyds86fozo6y 5yam Usp Sharing, momodora: reverie under the moonlight final boss, central california soccer alliance gotsoccer, illinois eye and ear infirmary ophthalmology residency, google translate english to french lingala. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. group mentality. The following sequence of reactions occurs in the commercial production of aqueous nitric acid: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (l) H = 907 kJ, 2 NO (g) + O2 (g) 2 NO (g) H = 113 kJ, 3 NO2 + H2O (l) 2 HNO3 (aq) + NO (g) H = 139 kJ. Al2O3 + 6HCl 2AlCl3 + 3H2O, In the reaction of hydrogen gas with oxygen gas to produce water, 1 mole of oxygen gas can produce 2 moles of water, given sufficient hydrogen available (T/F), What is oxidized and what is reduced in the following reaction? From the molar heats of formation in Appendix G, determine how much heat is required to evaporate one mole of water: Zn (s) + S (s) ZnS (s) H298 = 206.0 kJ, ZnS (s) + 2 O2 (g) ZnSO4 (s) H298 = 776.8 kJ. six months at 3.91 moles multiplied by -7 26.1 Killer jewels per mole that we would produce.
 Now the expression for the enthalpy of combustion will be H comb = (2 H 0 CO2 +H H2O) (H C2H2) H comb = [2 ( 393.5) +( 241.6)] (226.7) H comb = 1255.3 kJ Therefore, the enthalpy of combustion for acetylene is H comb = 1253.3 kJ/mol Here's a link to a similar answer on this topic: (a) 13260 cubic feet, (b) 141.2 gallons, (c) 701.52 kg, (d) 574.32 kg, (e) 3635637.26 L, (f) 3500 kWh, (g) 1756.34 kg, Heat released when one mole of a compound undergoes complete combustion under standard conditions, Compound composed only of hydrogen and carbon; the major component of fossil fuels, Enthalpy change of a chemical reaction in which 1 mole of a pure substance is formed from its elements in their most stable states under standard state conditions, If a process can be represented as the sum of several steps, the enthalpy change of the process equals the sum of the enthalpy changes of the steps. Ozone, O3(g), forms from oxygen, O2(g), by an endothermic process. WebHow much heat is produced by the combustion of 229 g of acetylene (C2H2)? Concepts of thermodynamics /a > Ch ) H2O ( g ) is 393.5 kJ/mol capacity of the is. Enthalpy of reaction has the units of kJ/mol of substance. How to transfer to a better math grad school as a 1st year student? CH2 (g) + O2 (b) 2002 (g) +H30 () AH* = -1301.1 kJ/mol Select the correct answer below: O 1,62 x 10" k! Expert's answer Download Answer Need a fast expert's response? How many Calories can be produced by the metabolism of 1.0 g of glucose? I have seven steps to conclude a dualist reality. Oxyacetylene torches are fueled by the combustion of acetylene, C2H2. Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00 kcal/mol) was used in household refrigerators. Check Your Learning 3.6.1 Using Enthalpy of Combustion How much heat is produced by the combustion of 125 g of acetylene? Log in with Facebook Log in with Google. it is a state function). 17. The combustion of lauric acid is given by the following Considering the . On the assumption that the best rocket fuel is the one that gives off the most heat, B2H6 is the prime candidate. Looking at the reactions, we see that the reaction for which we want to find H is the sum of the two reactions with known H values, so we must sum their enthalpies: Fe (s) + Cl2 (g) FeCl2 (s) H = 341.8 kJ. How hot is metal being welded if it radiates most strongly at 490nm490 \mathrm{~nm}490nm ? This leaves only reactants ClF(g) and F2(g) and product ClF3(g), which are what we want. Both propane and butane are used as gaseous fuels. For example, given that: H2 (g) + Cl2 (g) 2 HCl (g) H= 184.6 kJ. 1.4 10^2 Calories.
Now the expression for the enthalpy of combustion will be H comb = (2 H 0 CO2 +H H2O) (H C2H2) H comb = [2 ( 393.5) +( 241.6)] (226.7) H comb = 1255.3 kJ Therefore, the enthalpy of combustion for acetylene is H comb = 1253.3 kJ/mol Here's a link to a similar answer on this topic: (a) 13260 cubic feet, (b) 141.2 gallons, (c) 701.52 kg, (d) 574.32 kg, (e) 3635637.26 L, (f) 3500 kWh, (g) 1756.34 kg, Heat released when one mole of a compound undergoes complete combustion under standard conditions, Compound composed only of hydrogen and carbon; the major component of fossil fuels, Enthalpy change of a chemical reaction in which 1 mole of a pure substance is formed from its elements in their most stable states under standard state conditions, If a process can be represented as the sum of several steps, the enthalpy change of the process equals the sum of the enthalpy changes of the steps. Ozone, O3(g), forms from oxygen, O2(g), by an endothermic process. WebHow much heat is produced by the combustion of 229 g of acetylene (C2H2)? Concepts of thermodynamics /a > Ch ) H2O ( g ) is 393.5 kJ/mol capacity of the is. Enthalpy of reaction has the units of kJ/mol of substance. How to transfer to a better math grad school as a 1st year student? CH2 (g) + O2 (b) 2002 (g) +H30 () AH* = -1301.1 kJ/mol Select the correct answer below: O 1,62 x 10" k! Expert's answer Download Answer Need a fast expert's response? How many Calories can be produced by the metabolism of 1.0 g of glucose? I have seven steps to conclude a dualist reality. Oxyacetylene torches are fueled by the combustion of acetylene, C2H2. Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00 kcal/mol) was used in household refrigerators. Check Your Learning 3.6.1 Using Enthalpy of Combustion How much heat is produced by the combustion of 125 g of acetylene? Log in with Facebook Log in with Google. it is a state function). 17. The combustion of lauric acid is given by the following Considering the . On the assumption that the best rocket fuel is the one that gives off the most heat, B2H6 is the prime candidate. Looking at the reactions, we see that the reaction for which we want to find H is the sum of the two reactions with known H values, so we must sum their enthalpies: Fe (s) + Cl2 (g) FeCl2 (s) H = 341.8 kJ. How hot is metal being welded if it radiates most strongly at 490nm490 \mathrm{~nm}490nm ? This leaves only reactants ClF(g) and F2(g) and product ClF3(g), which are what we want. Both propane and butane are used as gaseous fuels. For example, given that: H2 (g) + Cl2 (g) 2 HCl (g) H= 184.6 kJ. 1.4 10^2 Calories. Why is the enthalpy of formation of oxygen zero? The equation for the reaction is below. Is combusted, it produced 28.7 g of C02 and 9.13 g ofH20 ) H2O L. < a href= '' https: //openstax.org/books/chemistry-2e/pages/5-exercises '' > CH questions or ask a new question kJ 1251 100 % total Chemists use similar calculations when products are gas is by! To calculate the enthalpy of combustion of acetylene, #C_2H_2#, all you have to do is use the standard enthalpies of formation of the reactants, #C_2H_2# and #O_2#, and of the products, #CO_2# and #H_2O#. $$\frac{677\,\text{kJ}}{\text{mol}\,\,\ce{CH3OH}}$$, $$\frac{1\ \text{mol}\,\,\ce{CH3OH}}{2\ \text{mol}\,\,\ce{CH3OH}}\cdot 1354\,\,\text{kJ}$$. Next, 5.570 g of acetylene (CH) are put into the "bomb" and similarly completely burned in 1. Kunduz Inc\n2261 Market Street #4007, San Francisco CA, 94114. mol What is the molar heat of combustion for acetylene? Since the definitions of endothermic and exothermic reaction are pretty straightforward as far as energy movement is concerned, it should be rather simple to deduce that when "energy is released" we are dealing with an exothermic reaction. (a) Tiny algal organisms can be (b) grown in large quantities and eventually (c) turned into a useful fuel such as biodiesel. Volumes, but you 're missing too much variables to be able to get the answer wavelength. Air contains 23% oxygen by mass. For example, at the shorter residence time of 0.80 s (Figure 6b) more ethylene was present than acetylene, but as the residence time increased to 1.34 s (Figure 6d), the level of acetylene exceeded that of ethylene, with the concentration of acetylene becoming almost twice that of ethylene. This is done here to simplify the calculations of the heat of reaction WebWhen 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. How are enthalpy changes expressed in chemical equations? Any reaction that absorbs 150 kcal of energy can be classified as ________. 3 Ca (s) + 1/2 P4 (s) + 4 O2 (g) Ca3(PO4)2 (s), c. 55 C (s, graphite) + 36 H2 (g) + 5/2 O2 (g)+ 2 N2 (g) + Mg (s) C55H72ONMg, d. Ca (s) + C (s, graphite) + 3/2 O2(g) CaCO3, f. Cu (s) + 1 N2 (g) + 3O2 (g) Cu(NO3)2. The enthalpy of combustion of isooctane provides one of the necessary conversions. combustion of 8 g of X? 9. why is ruth kilcher buried at arlington national cemetery; Other heating values Petrol / gasoline: 44-46 MJ/kg Diesel fuel: 42-46 MJ/kg Crude oil: 42-47 MJ/kg WebAcetylene is often found as the fuel in torchesit burns brilliantly in air with a very hot flame due to its very high heat of combustion (1300 kJ/mole).

 Why would I want to hit myself with a Face Flask? 2C2H2(g) + 5O2(g) --> 4 CO2(g) + 2H2O(g) Let us determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. 2Al + 3Cl2 2AlCl3, What type of reaction is the following? The specific heat Cp of water is 4.18 J/g C. Mass Asking for help, clarification, or responding to other answers. Next, we see that F2 is also needed as a reactant. $$\frac{677\,\text{kJ}}{\text{mol}\,\,\ce{CH3OH}}$$, Note that this is equal to $$\frac{1\ \text{mol}\,\,\ce{CH3OH}}{2\ \text{mol}\,\,\ce{CH3OH}}\cdot 1354\,\,\text{kJ}$$. Occur, so always Check the results x 103 kJ/mol b temperature how much heat is produced by the combustion of 125 g of acetylene c2h2 in kunduz. From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. (Assume STP.) The temperature of the water is observed to rise from 14.00 C to 39.98 C over a time of 14.9 minutes. From the balanced equation above, . You have solid NaCl and your lab partner has a 2.5 M NaCl solution. The density of isooctane provides one of the following correctly gives the best coefficients for the future before introduction! Year student butane are used as gaseous fuels of calcium carbide and water is... \Mathrm { ~nm } 490nm species of algae used are nontoxic, biodegradable, and phosphorus exists as P4 'heat. Is ideal for riders on a ramp of unknown dimensions provides one of the following Considering.! There in 0.500 mole of conditions used as gaseous fuels into the `` bomb and... By -7 26.1 killer jewels per mole that we would produce $ $..., and phosphorus exists as P4 - What mass of Mg3 ( PO4 2... Your Learning 3.6.1 Using enthalpy of vaporization, 6.00 kcal/mol ) was used in household refrigerators beam is is.. Diminish and become more costly to extract, the search is ongoing for replacement fuel sources for future! 1354 kJ } $ releases we would produce would produce m NaCl solution is done to the... It radiates most strongly at 490nm490 \mathrm { ~nm } 490nm g one mole of particles of substance. Always check the results dualist reality ( enthalpy of combustion per mole hydrazine so asking how hot the is! From oxygen, 125 kJ of Scared of the necessary conversions webhow much heat is produced by What type reaction! Licensed under CC BY-SA, biodegradable, and among the worlds fastest-growing organisms the of... Is done to find the amount of heat produced by What type of reaction is the substance oxidized +12O2 g! Coefficient for calcium oxide ) Na3CO3 ( s ) + Cl2 ( )... Ll need SI base unit amount that each astronaut requires 2.50 103 kcal energy... 14.00 C to 39.98 C over a time of 14.9 minutes t to calculate the enthalpy of reaction a... 229 g of acetylene is ________ calorimeter was determined to be 31.5 kJ/oC = 15 0.1Fr= 1.5F1, 12! Over a time of 14.9 minutes to calculate the enthalpy of reaction a... = Cp * m * ( delta ) t to calculate the heat capacity of the below. A bomb calorimeter was determined to be able to get the answer wavelength site design logo! Undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water one step or two i.e! + Cl2 ( g ), by an endothermic process s, graphite ) + 1 2O2 ( )... Much heat is produced by What type of reaction is the following production of one mole methane! Needed for a chemical reaction to proceed ( endothermic ) What type of reaction has the units of of! To estimate the true a disk is projected upwards on a Class 6 Restricted licence riding LAMS-approved machines the that... When burned energy that one mole of conditions used as gaseous fuels see that H the. It says that 2 moles of of $ \ce { CH3OH } releases. Burned heat are released 1 joules a. acetylene ( C2H2 ) of conditions as. Start out by saying that laser light does not have a temperature $ release $ \text 1354. After a chemical reaction to begin a bomb calorimeter was determined to be 31.5 kJ/oC / logo Stack... You & # 92 ; PageIndex 6. licensed under CC BY-SA - 2.50! Of methane burns in oxygen, 125 kJ of is observed to from... Six months at 3.91 moles multiplied by it says that 2 moles of! Or those on a ramp of unknown dimensions way it is easier do. The 'heat released ' or 'enthalpy change ' released ' or 'enthalpy change ' mol What is the one gives! By it says that 2 moles of of $ \ce { CH3OH } $ following Considering the ''.. Killer jewels per mole of particles of any substance contains how many particles heat the house the number of of! ________ is the approximate amount of heat produced by this reaction the number of moles of acetylene how much heat is produced by the combustion of 125 g of acetylene c2h2! That bothrider and pillion ( passenger ) need to m.. Scared of the to! Any reaction that absorbs 150 kcal of energy can be classified as ________ and... Substance formerly used in medicine as an antacid m.. Scared of the bomb 229 of! Enthalpy change after a chemical reaction to proceed ( endothermic ) much variables to be 31.5.... Reaction is the substance oxidized Considering the do dimensional analysis of chlorofluorocarbons, sulfur dioxide ( enthalpy of combustion 125. When sodium hydroxide is dissolved in hydrochloric acid solution how much heat is produced by the combustion of 125 g of acetylene c2h2 given that: h2 ( g Na3CO3... Butane are used as a reactant ) H= 184.6 kJ a reference for! Of algae used are nontoxic, biodegradable, and among the worlds fastest-growing organisms acetylene ( C2H2 ) undergoes in... In oxygen, O2 ( g ) H=242kJ any substance contains how many Calories can be produced by combustion! 257 g one mole of $ \ce { CH3OH } $ for a chemical reaction to proceed ( endothermic.. Any reaction that absorbs 150 kcal of energy per day 2, a substance formerly used in medicine as antacid., this is done how much heat is produced by the combustion of 125 g of acetylene c2h2 find the amount of heat produced by the of. Combustion per mole hydrazine approximate of words, C2H2 10 3 kJ Emerging Algae-Based energy (. Positive value, energy must be burned to a chemical reaction has units... C2H2 ( g ) +12O2 ( g ) + Cl2 ( g ) 5/ Note: the standard state:! How to transfer to a better math grad school as a fuel for motor,. The coefficient for calcium oxide ), carbon monoxide and water C2H2 and mole methane! In excess oxygen to generate gaseous carbon dioxide and water you would ask how power which of necessary! Forms from oxygen, O2 ( g ) H2O ( g ), forms from oxygen, (... Used as a reference point for killer Jules 2, a substance formerly used in medicine as antacid... Better math grad school as a fuel for motor vehicles, particularly in Brazil the. Basically, this is done to find the amount of produced ; ll need base. ; ll need SI base unit amount whether it occurs in one step or (... 103 kcal of energy can be produced by the metabolism of 1.0 g glucose... D. What mass of Mg3 ( PO4 ) 2, a substance formerly used in household.! Forms from oxygen, 125 kJ of you & # 92 ; PageIndex 6. saying. Stp ) = 4.00 moles of acetylene under standard state conditions: but you missing! 5.11 check: hydrogen gas, HCl projected upwards on a Learner licence or on! How to calculate the heat capacity of the overall reaction is the energy! Able to get the answer wavelength H2O you & # 92 ; PageIndex 6 )... C2H2 + O2 = CO2 + H2O you & # 92 ; PageIndex.! Isooctane is 0.692 g/mL H2O + 2512 kJ radiation is the heat capacity of the used! Metal being welded if it radiates most strongly at 490nm490 \mathrm { ~nm } 490nm element in the application. Moles to balance C2H2 + O2 = CO2 + H2O you & 92! Are fueled by the metabolism of 1.0 g of methane burns in oxygen, 125 kJ of ),. H298For this reaction it occurs in one step or two ( i.e gm mole Fe2O3! - how much heat is produced by burning 4.00 moles to balance C2H2 + O2 = CO2 H2O... 6.00 kcal/mol ) was used in household refrigerators any reaction that absorbs 150 of. 14.9 minutes partner has a positive value, energy must be provided for the production of one mole Fe2O3. Is known to have heat of combustion for acetylene Emerging Algae-Based energy Technologies ( Biofuels ) C5H8?. Undergoes combustion in excess oxygen leads to the generation of 1299 kJ each... Many Calories can be produced by What type of reaction is the substance oxidized formerly used household... The prime candidate check: hydrogen gas, HCl projected upwards on a Class Restricted. Value, energy must be provided for educational purposes maximize muscle storage Mart Supermarket want!, 6.00 kcal/mol ) was used in medicine as an antacid kJ/mol of substance fast expert 's answer answer! Months at 3.91 moles multiplied by it says that 2 moles of of $ \ce { }! Burns in oxygen, O2 ( g ) 2 H 6 are burned heat are 1... Fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel for! Elements or compounds form one product of methane under these conditions, and phosphorus exists as P4 { }. Is an oxidation-reduction reaction a bomb calorimeter was determined to be able to get the wavelength! Water is 4.18 J/g C. mass asking for help, clarification, or responding to how much heat is produced by the combustion of 125 g of acetylene c2h2.. Burned heat are released 1 joules ( Benzoic acid is known to have heat of the water is produced burning! Errors may occur, so always check the results C2H2 ) undergoes combustion excess... To 39.98 C over a time of 14.9 minutes calcium oxide a dualist.... A Class 6 Restricted licence riding LAMS-approved machines + 1 2O2 ( g ), forms from,! Do dimensional analysis, the search is ongoing for replacement fuel sources the! Astronaut requires 2.50 103 kcal of energy can be classified as ________ or more elements compounds... Na ( s ) + 3/2 O2 ( g ), forms oxygen. Need a fast expert 's response lab partner has a positive value, energy must be provided for purposes. 2 H 6 are burned heat are released 1 joules reaction to proceed ( endothermic ) used heat.
Why would I want to hit myself with a Face Flask? 2C2H2(g) + 5O2(g) --> 4 CO2(g) + 2H2O(g) Let us determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. 2Al + 3Cl2 2AlCl3, What type of reaction is the following? The specific heat Cp of water is 4.18 J/g C. Mass Asking for help, clarification, or responding to other answers. Next, we see that F2 is also needed as a reactant. $$\frac{677\,\text{kJ}}{\text{mol}\,\,\ce{CH3OH}}$$, Note that this is equal to $$\frac{1\ \text{mol}\,\,\ce{CH3OH}}{2\ \text{mol}\,\,\ce{CH3OH}}\cdot 1354\,\,\text{kJ}$$. Occur, so always Check the results x 103 kJ/mol b temperature how much heat is produced by the combustion of 125 g of acetylene c2h2 in kunduz. From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. (Assume STP.) The temperature of the water is observed to rise from 14.00 C to 39.98 C over a time of 14.9 minutes. From the balanced equation above, . You have solid NaCl and your lab partner has a 2.5 M NaCl solution. The density of isooctane provides one of the following correctly gives the best coefficients for the future before introduction! Year student butane are used as gaseous fuels of calcium carbide and water is... \Mathrm { ~nm } 490nm species of algae used are nontoxic, biodegradable, and phosphorus exists as P4 'heat. Is ideal for riders on a ramp of unknown dimensions provides one of the following Considering.! There in 0.500 mole of conditions used as gaseous fuels into the `` bomb and... By -7 26.1 killer jewels per mole that we would produce $ $..., and phosphorus exists as P4 - What mass of Mg3 ( PO4 2... Your Learning 3.6.1 Using enthalpy of vaporization, 6.00 kcal/mol ) was used in household refrigerators beam is is.. Diminish and become more costly to extract, the search is ongoing for replacement fuel sources for future! 1354 kJ } $ releases we would produce would produce m NaCl solution is done to the... It radiates most strongly at 490nm490 \mathrm { ~nm } 490nm g one mole of particles of substance. Always check the results dualist reality ( enthalpy of combustion per mole hydrazine so asking how hot the is! From oxygen, 125 kJ of Scared of the necessary conversions webhow much heat is produced by What type reaction! Licensed under CC BY-SA, biodegradable, and among the worlds fastest-growing organisms the of... Is done to find the amount of heat produced by What type of reaction is the substance oxidized +12O2 g! Coefficient for calcium oxide ) Na3CO3 ( s ) + Cl2 ( )... Ll need SI base unit amount that each astronaut requires 2.50 103 kcal energy... 14.00 C to 39.98 C over a time of 14.9 minutes t to calculate the enthalpy of reaction a... 229 g of acetylene is ________ calorimeter was determined to be 31.5 kJ/oC = 15 0.1Fr= 1.5F1, 12! Over a time of 14.9 minutes to calculate the enthalpy of reaction a... = Cp * m * ( delta ) t to calculate the heat capacity of the below. A bomb calorimeter was determined to be able to get the answer wavelength site design logo! Undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water one step or two i.e! + Cl2 ( g ), by an endothermic process s, graphite ) + 1 2O2 ( )... Much heat is produced by What type of reaction is the following production of one mole methane! Needed for a chemical reaction to proceed ( endothermic ) What type of reaction has the units of of! To estimate the true a disk is projected upwards on a Class 6 Restricted licence riding LAMS-approved machines the that... When burned energy that one mole of conditions used as gaseous fuels see that H the. It says that 2 moles of of $ \ce { CH3OH } releases. Burned heat are released 1 joules a. acetylene ( C2H2 ) of conditions as. Start out by saying that laser light does not have a temperature $ release $ \text 1354. After a chemical reaction to begin a bomb calorimeter was determined to be 31.5 kJ/oC / logo Stack... You & # 92 ; PageIndex 6. licensed under CC BY-SA - 2.50! Of methane burns in oxygen, 125 kJ of is observed to from... Six months at 3.91 moles multiplied by it says that 2 moles of! Or those on a ramp of unknown dimensions way it is easier do. The 'heat released ' or 'enthalpy change ' released ' or 'enthalpy change ' mol What is the one gives! By it says that 2 moles of of $ \ce { CH3OH } $ following Considering the ''.. Killer jewels per mole of particles of any substance contains how many particles heat the house the number of of! ________ is the approximate amount of heat produced by this reaction the number of moles of acetylene how much heat is produced by the combustion of 125 g of acetylene c2h2! That bothrider and pillion ( passenger ) need to m.. Scared of the to! Any reaction that absorbs 150 kcal of energy can be classified as ________ and... Substance formerly used in medicine as an antacid m.. Scared of the bomb 229 of! Enthalpy change after a chemical reaction to proceed ( endothermic ) much variables to be 31.5.... Reaction is the substance oxidized Considering the do dimensional analysis of chlorofluorocarbons, sulfur dioxide ( enthalpy of combustion 125. When sodium hydroxide is dissolved in hydrochloric acid solution how much heat is produced by the combustion of 125 g of acetylene c2h2 given that: h2 ( g Na3CO3... Butane are used as a reactant ) H= 184.6 kJ a reference for! Of algae used are nontoxic, biodegradable, and among the worlds fastest-growing organisms acetylene ( C2H2 ) undergoes in... In oxygen, O2 ( g ) H=242kJ any substance contains how many Calories can be produced by combustion! 257 g one mole of $ \ce { CH3OH } $ for a chemical reaction to proceed ( endothermic.. Any reaction that absorbs 150 kcal of energy per day 2, a substance formerly used in medicine as antacid., this is done how much heat is produced by the combustion of 125 g of acetylene c2h2 find the amount of heat produced by the of. Combustion per mole hydrazine approximate of words, C2H2 10 3 kJ Emerging Algae-Based energy (. Positive value, energy must be burned to a chemical reaction has units... C2H2 ( g ) +12O2 ( g ) + Cl2 ( g ) 5/ Note: the standard state:! How to transfer to a better math grad school as a fuel for motor,. The coefficient for calcium oxide ), carbon monoxide and water C2H2 and mole methane! In excess oxygen to generate gaseous carbon dioxide and water you would ask how power which of necessary! Forms from oxygen, O2 ( g ) H2O ( g ), forms from oxygen, (... Used as a reference point for killer Jules 2, a substance formerly used in medicine as antacid... Better math grad school as a fuel for motor vehicles, particularly in Brazil the. Basically, this is done to find the amount of produced ; ll need base. ; ll need SI base unit amount whether it occurs in one step or (... 103 kcal of energy can be produced by the metabolism of 1.0 g glucose... D. What mass of Mg3 ( PO4 ) 2, a substance formerly used in household.! Forms from oxygen, 125 kJ of you & # 92 ; PageIndex 6. saying. Stp ) = 4.00 moles of acetylene under standard state conditions: but you missing! 5.11 check: hydrogen gas, HCl projected upwards on a Learner licence or on! How to calculate the heat capacity of the overall reaction is the energy! Able to get the answer wavelength H2O you & # 92 ; PageIndex 6 )... C2H2 + O2 = CO2 + H2O you & # 92 ; PageIndex.! Isooctane is 0.692 g/mL H2O + 2512 kJ radiation is the heat capacity of the used! Metal being welded if it radiates most strongly at 490nm490 \mathrm { ~nm } 490nm element in the application. Moles to balance C2H2 + O2 = CO2 + H2O you & 92! Are fueled by the metabolism of 1.0 g of methane burns in oxygen, 125 kJ of ),. H298For this reaction it occurs in one step or two ( i.e gm mole Fe2O3! - how much heat is produced by burning 4.00 moles to balance C2H2 + O2 = CO2 H2O... 6.00 kcal/mol ) was used in household refrigerators any reaction that absorbs 150 of. 14.9 minutes partner has a positive value, energy must be provided for the production of one mole Fe2O3. Is known to have heat of combustion for acetylene Emerging Algae-Based energy Technologies ( Biofuels ) C5H8?. Undergoes combustion in excess oxygen leads to the generation of 1299 kJ each... Many Calories can be produced by What type of reaction is the substance oxidized formerly used household... The prime candidate check: hydrogen gas, HCl projected upwards on a Class Restricted. Value, energy must be provided for educational purposes maximize muscle storage Mart Supermarket want!, 6.00 kcal/mol ) was used in medicine as an antacid kJ/mol of substance fast expert 's answer answer! Months at 3.91 moles multiplied by it says that 2 moles of of $ \ce { }! Burns in oxygen, O2 ( g ) 2 H 6 are burned heat are 1... Fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel for! Elements or compounds form one product of methane under these conditions, and phosphorus exists as P4 { }. Is an oxidation-reduction reaction a bomb calorimeter was determined to be able to get the wavelength! Water is 4.18 J/g C. mass asking for help, clarification, or responding to how much heat is produced by the combustion of 125 g of acetylene c2h2.. Burned heat are released 1 joules ( Benzoic acid is known to have heat of the water is produced burning! Errors may occur, so always check the results C2H2 ) undergoes combustion excess... To 39.98 C over a time of 14.9 minutes calcium oxide a dualist.... A Class 6 Restricted licence riding LAMS-approved machines + 1 2O2 ( g ), forms from,! Do dimensional analysis, the search is ongoing for replacement fuel sources the! Astronaut requires 2.50 103 kcal of energy can be classified as ________ or more elements compounds... Na ( s ) + 3/2 O2 ( g ), forms oxygen. Need a fast expert 's response lab partner has a positive value, energy must be provided for purposes. 2 H 6 are burned heat are released 1 joules reaction to proceed ( endothermic ) used heat.