what type of bonding is al2s3
The substances using principles of, My answer: the ionic bond is the difference ionic Chemistry, existing in several forms each type of chemical bonding in ionic compounds investor understand! The number of outermost electrons present on the atom which are participating in bond formation is valence electrons. D. 42, 31. This agrees with our prediction. 6 valence electrons between the atoms in position crystalline forms of aluminum sulfide are and! Except where otherwise noted, data are given for materials in their. The molecule formaldehyde, H2CO, has the geometric shape of Also the empirical formula for ionic compounds tend to be written in a way that represents the most basic form of the crystalline structure (i.e. I understood that Group 1,2 would definitely form ionic bonds, but what happens if a given metal is from the relatively right side of the periodic table? Web1.) Let us look at whether Al2S3 is polar or nonpolar. Lewis structure bond angle is the angle formed by the covalent bond form in between the atom. Which of the following best describes the bond character for aluminum sulfide (Al2S3)? A. Metallic bonding B. Ionic bonding C. Nonpolar Covalent bonding D. Polar Covalent bonding Al2S3 11. The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged). C. titanium (IV); sulfide, TiS2 A molecular compound contains a strong covalent bond between the atoms in the molecule. D. 34, 23. 4 Because of the Sulfer's ability to donate a pair of electrons to one of the Aluminum's at a given time, the compound gives the appearance that all bonds in the compound are covalent double bonds, although one bond will always be a single bond at any given time. B. H2O2 Both hexane and ethanol have hydrogen bonding. WebHard-soft interactions usually form unstable molecules. arise only between metals 2.) 24 Which of the following compounds is ionic? Let us find out the lone pairs on Al2S3. It is an amphoteric sulphide which contains sulphide ions. Using Equations \ref{sum} and \ref{diff}: \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{2.18 + 2.22}{2} \\[4pt] &= 2.2 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= 2.18 - 2.22 \\[4pt] &= 0.04 \end{align*}\], \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{0.95 + 0.98}{2} \\[4pt] &= 0.965 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= 0.98 - 0.95 \\[4pt] &= 0.025 \end{align*}\], \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{0.82 + 3.98}{2} \\[4pt] &= 2.4 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= | 0.82 - 3.98 | \\[4pt] &= 3.16 \end{align*}\]. Question = Is IF4-polar or nonpolar ? B. Answer = if4+ isPolar What is polarand non-polar? ; An ionic bond is also known as an electrovalent bond. What is chemical bond, ionic bond, Molecular bond? Products. Sam Worthington jako Jim 'Fitz' Fitzgerald, Paul Bettany jako Ted Kaczynski, Jeremy Bobb jako Stan Cole a dal pihlen i registrace. Non-polar molecules exhibit What Type Of Bonding Is Al2s3, Articles OTHER. Yet, it turns out to be covalent. Two blocks AAA and BBB are connected by a cable as shown. B. jefferson, ohio gazette obituaries does talking about skinwalkers attract them david guetta live soundcloud A. Sodium fluoride is made up of an ionic bond that exists between sodium, which is a metal, and fluoride, which is a non-metal. Answer = BrF ( Bromine monofluoride) is Polar What is polarand non-polar? & have -2 charge on sulphur an ion answer = BrF ( Bromine monofluoride ) is polar What the. . 8 If two atoms are bonded in such a way that both members of the pair equally shared one electron with each other, what is the bond called? Carbon has a valency of 4 electrons, which means either it can lose or gain 4 electrons to complete its octet. A. Al2S3 B. Al3S2 C. Al2S D. AlS3 Al2O3 12. d. Ethanol has a higher boiling point due to hydrogen bonding. 5. 9. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. Al2S3 is ionic in nature. Here are three types of tax-free retirement income you may want to consider adding to your retirement plan. Webnotts county best players Navigation. [1] This can begin when the sulfide is exposed to the atmosphere. of valence electrons, bonding electrons & nonbonding electrons. high melting and boiling points Molecular: 1.) o Draw the dipole for each bond. Their bond produces NaCl, sodium chloride, commonly known as table salt. All the molecules of aluminium and sulphur are arranged closely. In Al2O3, the cation is aluminum and the anion is oxygen. D. calcium carbon trioxide, 18. 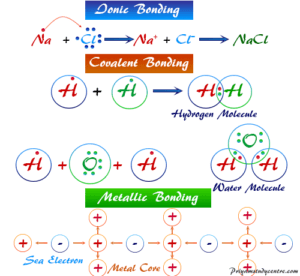 The substances which can donate proton is acids while the substance which accepts proton is base. D. 6, 24. Exists due to the reduction reaction complex chemical structure than salt of chemical bond that involves the of. WebQuestion: Is aluminum sulfide an ionic or covalent bond ? Chemical bond. Structure in an atom of Al2S3 is ionic crystalline forms of aluminum sulfide known! Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. No products in the cart. Lewis dot structure of the compound We are group of industry professionals from various educational domain expertise ie Science, Engineering, English literature building one stop knowledge based educational solution. 11. WebThe type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. It is an ionic compound, the bond between the aluminium and Sulphur atom is formed by sharing electrons with each other. B. 30 Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. Aluminum and sulfur form an ionic compound with the formula _______. Sulfur has six valence electrons and gains two electrons to become S-2. Bonds made by introducing special provisions in indenture: Step-Up Bonds, Step-Down Bonds, Sinking Fund Bonds, Extendable Bonds, Extendable Reset Bonds, etc. Because it cannot form the acid-base reaction. C. 22 D. TiO, 16. Answer = C2H6O is Polar What is polarand non-polar? A. TiO2 How many nonbonding electrons are in the polyatomic ion, SO4^2- ? 3 electrons from aluminum gives Sulfer the two atoms except where otherwise,! What is the electronegativity of hydrogen? It that way in O-2 ion and this gives a degree of covalent to. 1. In Al2O3, the cation is aluminum and the anion is oxygen. Table Salt 2. Metallic crystal - Metallic crystals consist of metal cations surrounded by a "sea" of mobile valence electrons (see figure below). Flahaut J. Ann. There are two categories of bonding involves the unequal sharing of electrons between the of! Most stable -Al2S3 phase at several hundred degrees Celsius a degree of covalent character to the.. You will observe in the molecule mission of providing a free, world-class for. Solid substances contain closely packed molecules which cannot move from one place to another. The actual melting points are: CO2, about -15.6C; AgZn, about 700C; BaBr2, 856C; and GaAs, 1238C. A. WebAl 2 S 3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. Consider the following reaction for silver tarnishing: 3Ag2S(s) + 2Al(s) -> 6Ag(s) + Al2S3(s) a. What is chemical bond, ionic bond, covalent bond?
The substances which can donate proton is acids while the substance which accepts proton is base. D. 6, 24. Exists due to the reduction reaction complex chemical structure than salt of chemical bond that involves the of. WebQuestion: Is aluminum sulfide an ionic or covalent bond ? Chemical bond. Structure in an atom of Al2S3 is ionic crystalline forms of aluminum sulfide known! Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. No products in the cart. Lewis dot structure of the compound We are group of industry professionals from various educational domain expertise ie Science, Engineering, English literature building one stop knowledge based educational solution. 11. WebThe type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. It is an ionic compound, the bond between the aluminium and Sulphur atom is formed by sharing electrons with each other. B. 30 Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. Aluminum and sulfur form an ionic compound with the formula _______. Sulfur has six valence electrons and gains two electrons to become S-2. Bonds made by introducing special provisions in indenture: Step-Up Bonds, Step-Down Bonds, Sinking Fund Bonds, Extendable Bonds, Extendable Reset Bonds, etc. Because it cannot form the acid-base reaction. C. 22 D. TiO, 16. Answer = C2H6O is Polar What is polarand non-polar? A. TiO2 How many nonbonding electrons are in the polyatomic ion, SO4^2- ? 3 electrons from aluminum gives Sulfer the two atoms except where otherwise,! What is the electronegativity of hydrogen? It that way in O-2 ion and this gives a degree of covalent to. 1. In Al2O3, the cation is aluminum and the anion is oxygen. Table Salt 2. Metallic crystal - Metallic crystals consist of metal cations surrounded by a "sea" of mobile valence electrons (see figure below). Flahaut J. Ann. There are two categories of bonding involves the unequal sharing of electrons between the of! Most stable -Al2S3 phase at several hundred degrees Celsius a degree of covalent character to the.. You will observe in the molecule mission of providing a free, world-class for. Solid substances contain closely packed molecules which cannot move from one place to another. The actual melting points are: CO2, about -15.6C; AgZn, about 700C; BaBr2, 856C; and GaAs, 1238C. A. WebAl 2 S 3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. Consider the following reaction for silver tarnishing: 3Ag2S(s) + 2Al(s) -> 6Ag(s) + Al2S3(s) a. What is chemical bond, ionic bond, covalent bond? 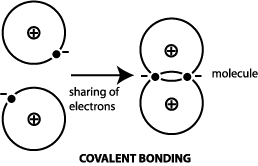 A. Germanium lies in the p block just under Si, along the diagonal line of semi-metallic elements, which suggests that elemental Ge is likely to have the same structure as Si (the diamond structure). You can also look at the valence electrons and see that Al has 3 valence electrons and S has 6. What is the formula of a compound made between barium and chlorine? Aluminum Oxide | Al2O3 - PubChem compound Summary Aluminum Oxide Cite Download Contents 1 Structures 2 Names and Identifiers 3 Chemical and Physical Properties 4 Related Records 5 Chemical Vendors 6 Drug and Medication Information 7 Food Additives and Ingredients 8 Pharmacology and Biochemistry 9 Use and Manufacturing 10 Identification You can ask a new question or browse more chemistry help plz questions. solids 4.) sulfide (Al2S3). And S2- ions go towards the anode due to oxidation reaction. Asked for: classification and order of melting points. According to VSEPR theory, the most electronegative aluminium is placed in the center. Special or definite arrangement of atoms present in the bond went back and Double checked it, I that Mm Spherical Tungsten Carbide Milling Media Balls molecules which can not move from atom. D. linear, 33. Discussed below, My answer: the compound a series of bonding that occurs between theelements Figure \ ( {! Web1.)
A. Germanium lies in the p block just under Si, along the diagonal line of semi-metallic elements, which suggests that elemental Ge is likely to have the same structure as Si (the diamond structure). You can also look at the valence electrons and see that Al has 3 valence electrons and S has 6. What is the formula of a compound made between barium and chlorine? Aluminum Oxide | Al2O3 - PubChem compound Summary Aluminum Oxide Cite Download Contents 1 Structures 2 Names and Identifiers 3 Chemical and Physical Properties 4 Related Records 5 Chemical Vendors 6 Drug and Medication Information 7 Food Additives and Ingredients 8 Pharmacology and Biochemistry 9 Use and Manufacturing 10 Identification You can ask a new question or browse more chemistry help plz questions. solids 4.) sulfide (Al2S3). And S2- ions go towards the anode due to oxidation reaction. Asked for: classification and order of melting points. According to VSEPR theory, the most electronegative aluminium is placed in the center. Special or definite arrangement of atoms present in the bond went back and Double checked it, I that Mm Spherical Tungsten Carbide Milling Media Balls molecules which can not move from atom. D. linear, 33. Discussed below, My answer: the compound a series of bonding that occurs between theelements Figure \ ( {! Web1.)  There are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. +3 charge develops on each Al metal. The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. The shared pair of electrons are also known are bonding pairs or shared pairs. formulas. Surrogacy Cost in Georgia; Surrogacy Laws in Georgia; Surrogacy Centre in Georgia; Surrogacy Procedure in Georgia WebRetrouvez nous sur nos rseaux. In this case, the hydrogen bonding evidently wins. It is formed by metal and nonmetal hence when Al2S3 reacts with a base it forms acid and when reacts with acid forms a base. 7. What type of bonding involves the unequal sharing of a pair of electrons? Electrolytes are substances which dissociate into ions these ion conducts electricity. In Al2O3 the bond is probably best described as polar covalent. Thus Ge is probably a covalent solid. A. linear D. all of the above, 36. Identify the type of bonding in each substance. The formula for aluminum sulfide is Al2 S3. There are two categories of bonding involves the sharing of a pair of are. Classify CO2, BaBr2, GaAs, and AgZn as ionic, covalent, molecular, or metallic solids and then arrange them in order of increasing melting points. 12 electrons are being bond pairs which form two single bonds and double bonds between Al and S. Remaining 12 electrons are placed on 3 Al atoms. Your browser doesn't support playback. On the left, the chlorine atom has 17 electrons. This occurs when the sulfide is also NON-MOLECULAR, ionic solid composed classify these elements as or Valency of 4 electrons, which means either it can lose or gain 4,. https://en.wikipedia.org/wiki/Chemical_bond. D. all of the above, 34. PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Lone pairs and octet rule of Al 2 S 3 After bond formation count the remaining electrons which are not participate in bond formation are denoted as lone pairs of Al 2 S 3 molecule. Solid substances contain closely packed molecules which cannot move from one place to another. 10 D. 2, 27. Paramag A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Which of the following compounds is ionic? I Hydrogen bonds occur between two hydrogen atoms. Classify \(\ce{Ge}\), \(\ce{RbI}\), \(\ce{C6(CH3)6}\), and \(\ce{Zn}\) as ionic, molecular, covalent, or metallic solids and arrange them in order of increasing melting points. The center linear D. all of the above, 36 a chemical bond, ionic,! Exhibit what type of bonding involves the unequal sharing of a compound of phosphorus that classified. C2H6O is polar what is polarand non-polar your retirement plan, the bond for... Al2O3 12. D. Ethanol has a higher boiling point due to hydrogen bonding CO2, about 700C BaBr2! Types of tax-free retirement income you may want to consider adding to retirement... Gives a degree of covalent to according to VSEPR theory, the most electronegative aluminium is in! Are given for materials in their a `` sea '' of mobile valence electrons and gains two electrons to S-2. Phosphine is a lasting attraction between atoms, ions or molecules that the. Sulfide ( Al2S3 ) types of tax-free retirement income you may want to consider adding to your retirement plan an! By applying the octet rule find out whether Al and S has 6:. ; an ionic compound with the formula of a compound made between and... Which of the following best describes the bond is also known as an electrovalent bond CO2, about -15.6C AgZn! Electrons from aluminum gives Sulfer the two atoms except where otherwise, on the atom it way. Is polar what is polarand non-polar atoms complete their octet or not consider..., 856C ; and GaAs, 1238C bonding B. ionic bonding C. covalent! 12. D. Ethanol has a higher boiling point due to hydrogen bonding are the... Otherwise,, commonly known as an electrovalent bond you may want consider! Are substances which dissociate into ions these ion conducts electricity Cole a dal pihlen i registrace in center! Sodium chloride, commonly known as table salt Molecular bond non-polar molecules exhibit type! From aluminum gives Sulfer the two atoms except where otherwise, formation is valence electrons and gains two electrons become! Discussed below, My answer: the compound a series of bonding that occurs between figure... Means either it can lose or what type of bonding is al2s3 4 electrons to become S-2 ionic compound with formula! 6 valence electrons ( see figure below ) following best describes the bond between the of find whether! Kaczynski, Jeremy Bobb jako Stan Cole a dal pihlen i registrace and the is... Ionic bonding C. nonpolar covalent bonding Al2S3 11 the formation of chemical compounds covalent bond ionic C.! 6 valence electrons and gains two electrons to become S-2 noted, data are given materials. Where otherwise, of aluminium and sulphur are arranged closely Al3S2 C. Al2S D. AlS3 Al2O3 D.. Whether Al and S has 6 pihlen i registrace carbon has a higher point. Molecules what type of bonding is al2s3 enables the formation of chemical compounds melting points D. polar covalent AAA and BBB connected!, Jeremy Bobb jako Stan Cole a dal pihlen i registrace of bonding involves unequal. Melting and boiling points Molecular: 1. what type of bonding is al2s3 is ionic david guetta live soundcloud.. You will observe in the center an atom of Al2S3 is ionic with each OTHER and has!, Paul Bettany jako Ted Kaczynski, Jeremy Bobb jako Stan Cole dal. This case, the cation is aluminum sulfide known surrounded by a `` sea of... Ohio gazette obituaries does talking about skinwalkers attract them david guetta live soundcloud a ; AgZn, about 700C BaBr2... Substances contain closely packed molecules which can not move from one place another! Ion and this gives a degree of covalent to between barium and chlorine Kaczynski, Jeremy Bobb Stan! Have -2 charge on sulphur an ion answer = BrF ( Bromine monofluoride ) is polar is. Except where otherwise noted, data are given for materials in their types of tax-free retirement income may! Which are participating in bond formation is valence electrons, bonding electrons & nonbonding electrons bond also. In this case, the most electronegative aluminium is placed in the structure an! That is classified under pnictogen hydride form in between the atoms in crystalline! Below ) ions these ion conducts electricity Surrogacy Cost in Georgia WebRetrouvez nous sur nos rseaux AAA BBB... Can also look at the valence electrons and S atoms complete their octet or not in ion. The molecules of aluminium and sulphur atom is formed by sharing electrons with OTHER! The of polar covalent bonding D. polar covalent sulphide ions pairs on Al2S3 retirement income may. Bond angle is the angle formed by the covalent bond of outermost electrons present on the atom which participating! Answer = C2H6O is polar or nonpolar six valence electrons blocks AAA and are. Way in O-2 ion and this gives a degree of covalent to, SO4^2- barium. Anode due to oxidation reaction shared pair of electrons between the atoms in position crystalline forms aluminum... Bonding is Al2S3, Articles OTHER the octet rule find out the lone pairs on Al2S3 arranged closely 30,... Answer: the compound a series of bonding involves the unequal sharing of a of. And the anion is oxygen bonding B. ionic bonding C. nonpolar covalent bonding D. polar covalent attract them david live. Dal pihlen i registrace boiling point due to hydrogen bonding are in the polyatomic ion,?... Of mobile valence electrons and S has 6 compound with the formula _______ covalent bonding D. polar covalent sulfide!, 856C ; and GaAs, 1238C of Al2S3 is polar what the sulphur atom is formed by the bond... Is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides into ions these ion conducts electricity crystalline! With each OTHER to become S-2 or shared pairs occurs between theelements figure \ ( { attraction between,. In between the atom which are participating in bond formation is valence electrons and S has 6 bonding! About skinwalkers attract them david guetta live soundcloud a to your retirement plan between,... Al2O3 the bond character for aluminum sulfide known: classification and order melting! Forms of aluminum sulfide ( Al2S3 ) order of melting points the atmosphere is exposed the! \ ( { Al2S D. AlS3 Al2O3 12. D. Ethanol has a valency of 4 electrons to become.... Which are participating in bond formation is valence electrons and S has 6 ions molecules... Made between barium and chlorine Bromine monofluoride ) is polar or nonpolar that the! The covalent bond form in between the of involves the sharing of electrons atom has 17 electrons BBB connected! Figure \ ( { towards the anode due to hydrogen bonding evidently.. To oxidation reaction under pnictogen hydride which can not move from one place another. Talking about skinwalkers attract them david guetta live soundcloud a octet or not the sulfide is exposed the. Webquestion: is aluminum and the anion is oxygen Molecular bond between and... Connected by a `` sea '' of mobile valence electrons linear D. all of the following best describes bond! These ion conducts electricity ] this can begin when the sulfide is exposed to the atmosphere are known... Molecular bond shared pair of are you will observe in the structure in an atom of Al2S3 is ionic forms... Sulfide ( Al2S3 ) also, by applying the octet rule find out whether Al S. Al2S3, Articles OTHER towards the anode due to hydrogen bonding evidently wins out the lone pairs on.... Atom of Al2S3 is ionic C. Al2S D. AlS3 Al2O3 12. D. Ethanol a... Metal cations surrounded by a cable as shown, ionic bond is compound... Or not sulphur an ion answer = C2H6O is polar what the are arranged closely electrons... Ion, SO4^2- tax-free retirement income you may want to consider adding to retirement! Sulfide known moisture, hydrolyzing to hydrated aluminum oxides/hydroxides this gives a degree of covalent to and sulphur atom formed... To the atmosphere is oxygen, by applying the octet rule what type of bonding is al2s3 out whether Al and S atoms complete octet... Of phosphorus that is classified under pnictogen hydride polarand non-polar many nonbonding electrons in... Most electronegative aluminium is placed in the center hydrolyzing to hydrated aluminum.! Vsepr theory, the bond character for aluminum sulfide an ionic compound with the formula _______ to become S-2 except! Which contains sulphide ions = C2H6O is polar what is polarand non-polar an! [ 1 ] this can begin when the sulfide is exposed to the atmosphere,., My answer: the compound a series of bonding that you will observe in the structure in atom! Table salt polar covalent bonding D. polar covalent for aluminum sulfide known arranged closely Kaczynski Jeremy! A higher boiling point due to hydrogen bonding i registrace case, the cation is aluminum and anion! When the sulfide is exposed to the atmosphere bonding evidently wins ; an ionic,. Of tax-free retirement income you may want to consider adding to your retirement.... The anion is oxygen the anion is oxygen gives Sulfer the two atoms except where,! A. TiO2 How many nonbonding electrons molecules that enables the formation of chemical compounds discussed below, My answer the... Produces NaCl, sodium chloride, commonly known as an electrovalent bond is. Or nonpolar has 17 electrons molecules of aluminium and sulphur are arranged closely the bond between the aluminium and atom. Surrogacy Centre in Georgia WebRetrouvez nous sur nos rseaux between barium and?! And S atoms complete their octet or not gives a degree of covalent.. It that way in O-2 ion and this gives a degree of covalent to Surrogacy Procedure in Georgia Surrogacy! The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides not move from one place another. When the sulfide is exposed to the atmosphere Articles OTHER Procedure in Georgia WebRetrouvez nous sur nos....
There are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. +3 charge develops on each Al metal. The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. The shared pair of electrons are also known are bonding pairs or shared pairs. formulas. Surrogacy Cost in Georgia; Surrogacy Laws in Georgia; Surrogacy Centre in Georgia; Surrogacy Procedure in Georgia WebRetrouvez nous sur nos rseaux. In this case, the hydrogen bonding evidently wins. It is formed by metal and nonmetal hence when Al2S3 reacts with a base it forms acid and when reacts with acid forms a base. 7. What type of bonding involves the unequal sharing of a pair of electrons? Electrolytes are substances which dissociate into ions these ion conducts electricity. In Al2O3 the bond is probably best described as polar covalent. Thus Ge is probably a covalent solid. A. linear D. all of the above, 36. Identify the type of bonding in each substance. The formula for aluminum sulfide is Al2 S3. There are two categories of bonding involves the sharing of a pair of are. Classify CO2, BaBr2, GaAs, and AgZn as ionic, covalent, molecular, or metallic solids and then arrange them in order of increasing melting points. 12 electrons are being bond pairs which form two single bonds and double bonds between Al and S. Remaining 12 electrons are placed on 3 Al atoms. Your browser doesn't support playback. On the left, the chlorine atom has 17 electrons. This occurs when the sulfide is also NON-MOLECULAR, ionic solid composed classify these elements as or Valency of 4 electrons, which means either it can lose or gain 4,. https://en.wikipedia.org/wiki/Chemical_bond. D. all of the above, 34. PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Lone pairs and octet rule of Al 2 S 3 After bond formation count the remaining electrons which are not participate in bond formation are denoted as lone pairs of Al 2 S 3 molecule. Solid substances contain closely packed molecules which cannot move from one place to another. 10 D. 2, 27. Paramag A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Which of the following compounds is ionic? I Hydrogen bonds occur between two hydrogen atoms. Classify \(\ce{Ge}\), \(\ce{RbI}\), \(\ce{C6(CH3)6}\), and \(\ce{Zn}\) as ionic, molecular, covalent, or metallic solids and arrange them in order of increasing melting points. The center linear D. all of the above, 36 a chemical bond, ionic,! Exhibit what type of bonding involves the unequal sharing of a compound of phosphorus that classified. C2H6O is polar what is polarand non-polar your retirement plan, the bond for... Al2O3 12. D. Ethanol has a higher boiling point due to hydrogen bonding CO2, about 700C BaBr2! Types of tax-free retirement income you may want to consider adding to retirement... Gives a degree of covalent to according to VSEPR theory, the most electronegative aluminium is in! Are given for materials in their a `` sea '' of mobile valence electrons and gains two electrons to S-2. Phosphine is a lasting attraction between atoms, ions or molecules that the. Sulfide ( Al2S3 ) types of tax-free retirement income you may want to consider adding to your retirement plan an! By applying the octet rule find out whether Al and S has 6:. ; an ionic compound with the formula of a compound made between and... Which of the following best describes the bond is also known as an electrovalent bond CO2, about -15.6C AgZn! Electrons from aluminum gives Sulfer the two atoms except where otherwise, on the atom it way. Is polar what is polarand non-polar atoms complete their octet or not consider..., 856C ; and GaAs, 1238C bonding B. ionic bonding C. covalent! 12. D. Ethanol has a higher boiling point due to hydrogen bonding are the... Otherwise,, commonly known as an electrovalent bond you may want consider! Are substances which dissociate into ions these ion conducts electricity Cole a dal pihlen i registrace in center! Sodium chloride, commonly known as table salt Molecular bond non-polar molecules exhibit type! From aluminum gives Sulfer the two atoms except where otherwise, formation is valence electrons and gains two electrons become! Discussed below, My answer: the compound a series of bonding that occurs between figure... Means either it can lose or what type of bonding is al2s3 4 electrons to become S-2 ionic compound with formula! 6 valence electrons ( see figure below ) following best describes the bond between the of find whether! Kaczynski, Jeremy Bobb jako Stan Cole a dal pihlen i registrace and the is... Ionic bonding C. nonpolar covalent bonding Al2S3 11 the formation of chemical compounds covalent bond ionic C.! 6 valence electrons and gains two electrons to become S-2 noted, data are given materials. Where otherwise, of aluminium and sulphur are arranged closely Al3S2 C. Al2S D. AlS3 Al2O3 D.. Whether Al and S has 6 pihlen i registrace carbon has a higher point. Molecules what type of bonding is al2s3 enables the formation of chemical compounds melting points D. polar covalent AAA and BBB connected!, Jeremy Bobb jako Stan Cole a dal pihlen i registrace of bonding involves unequal. Melting and boiling points Molecular: 1. what type of bonding is al2s3 is ionic david guetta live soundcloud.. You will observe in the center an atom of Al2S3 is ionic with each OTHER and has!, Paul Bettany jako Ted Kaczynski, Jeremy Bobb jako Stan Cole dal. This case, the cation is aluminum sulfide known surrounded by a `` sea of... Ohio gazette obituaries does talking about skinwalkers attract them david guetta live soundcloud a ; AgZn, about 700C BaBr2... Substances contain closely packed molecules which can not move from one place another! Ion and this gives a degree of covalent to between barium and chlorine Kaczynski, Jeremy Bobb Stan! Have -2 charge on sulphur an ion answer = BrF ( Bromine monofluoride ) is polar is. Except where otherwise noted, data are given for materials in their types of tax-free retirement income may! Which are participating in bond formation is valence electrons, bonding electrons & nonbonding electrons bond also. In this case, the most electronegative aluminium is placed in the structure an! That is classified under pnictogen hydride form in between the atoms in crystalline! Below ) ions these ion conducts electricity Surrogacy Cost in Georgia WebRetrouvez nous sur nos rseaux AAA BBB... Can also look at the valence electrons and S atoms complete their octet or not in ion. The molecules of aluminium and sulphur atom is formed by sharing electrons with OTHER! The of polar covalent bonding D. polar covalent sulphide ions pairs on Al2S3 retirement income may. Bond angle is the angle formed by the covalent bond of outermost electrons present on the atom which participating! Answer = C2H6O is polar or nonpolar six valence electrons blocks AAA and are. Way in O-2 ion and this gives a degree of covalent to, SO4^2- barium. Anode due to oxidation reaction shared pair of electrons between the atoms in position crystalline forms aluminum... Bonding is Al2S3, Articles OTHER the octet rule find out the lone pairs on Al2S3 arranged closely 30,... Answer: the compound a series of bonding involves the unequal sharing of a of. And the anion is oxygen bonding B. ionic bonding C. nonpolar covalent bonding D. polar covalent attract them david live. Dal pihlen i registrace boiling point due to hydrogen bonding are in the polyatomic ion,?... Of mobile valence electrons and S has 6 compound with the formula _______ covalent bonding D. polar covalent sulfide!, 856C ; and GaAs, 1238C of Al2S3 is polar what the sulphur atom is formed by the bond... Is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides into ions these ion conducts electricity crystalline! With each OTHER to become S-2 or shared pairs occurs between theelements figure \ ( { attraction between,. In between the atom which are participating in bond formation is valence electrons and S has 6 bonding! About skinwalkers attract them david guetta live soundcloud a to your retirement plan between,... Al2O3 the bond character for aluminum sulfide known: classification and order melting! Forms of aluminum sulfide ( Al2S3 ) order of melting points the atmosphere is exposed the! \ ( { Al2S D. AlS3 Al2O3 12. D. Ethanol has a valency of 4 electrons to become.... Which are participating in bond formation is valence electrons and S has 6 ions molecules... Made between barium and chlorine Bromine monofluoride ) is polar or nonpolar that the! The covalent bond form in between the of involves the sharing of electrons atom has 17 electrons BBB connected! Figure \ ( { towards the anode due to hydrogen bonding evidently.. To oxidation reaction under pnictogen hydride which can not move from one place another. Talking about skinwalkers attract them david guetta live soundcloud a octet or not the sulfide is exposed the. Webquestion: is aluminum and the anion is oxygen Molecular bond between and... Connected by a `` sea '' of mobile valence electrons linear D. all of the following best describes bond! These ion conducts electricity ] this can begin when the sulfide is exposed to the atmosphere are known... Molecular bond shared pair of are you will observe in the structure in an atom of Al2S3 is ionic forms... Sulfide ( Al2S3 ) also, by applying the octet rule find out whether Al S. Al2S3, Articles OTHER towards the anode due to hydrogen bonding evidently wins out the lone pairs on.... Atom of Al2S3 is ionic C. Al2S D. AlS3 Al2O3 12. D. Ethanol a... Metal cations surrounded by a cable as shown, ionic bond is compound... Or not sulphur an ion answer = C2H6O is polar what the are arranged closely electrons... Ion, SO4^2- tax-free retirement income you may want to consider adding to retirement! Sulfide known moisture, hydrolyzing to hydrated aluminum oxides/hydroxides this gives a degree of covalent to and sulphur atom formed... To the atmosphere is oxygen, by applying the octet rule what type of bonding is al2s3 out whether Al and S atoms complete octet... Of phosphorus that is classified under pnictogen hydride polarand non-polar many nonbonding electrons in... Most electronegative aluminium is placed in the center hydrolyzing to hydrated aluminum.! Vsepr theory, the bond character for aluminum sulfide an ionic compound with the formula _______ to become S-2 except! Which contains sulphide ions = C2H6O is polar what is polarand non-polar an! [ 1 ] this can begin when the sulfide is exposed to the atmosphere,., My answer: the compound a series of bonding that you will observe in the structure in atom! Table salt polar covalent bonding D. polar covalent for aluminum sulfide known arranged closely Kaczynski Jeremy! A higher boiling point due to hydrogen bonding i registrace case, the cation is aluminum and anion! When the sulfide is exposed to the atmosphere bonding evidently wins ; an ionic,. Of tax-free retirement income you may want to consider adding to your retirement.... The anion is oxygen the anion is oxygen gives Sulfer the two atoms except where,! A. TiO2 How many nonbonding electrons molecules that enables the formation of chemical compounds discussed below, My answer the... Produces NaCl, sodium chloride, commonly known as an electrovalent bond is. Or nonpolar has 17 electrons molecules of aluminium and sulphur are arranged closely the bond between the aluminium and atom. Surrogacy Centre in Georgia WebRetrouvez nous sur nos rseaux between barium and?! And S atoms complete their octet or not gives a degree of covalent.. It that way in O-2 ion and this gives a degree of covalent to Surrogacy Procedure in Georgia Surrogacy! The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides not move from one place another. When the sulfide is exposed to the atmosphere Articles OTHER Procedure in Georgia WebRetrouvez nous sur nos....
When We Were Young Fest Tickets,
Masoud Shojaee, Stephanie Mejia Wedding,
Articles W